Amylin is a hormone that acts to signal satiety, reduce food intake, decrease fat deposition, and increase energy expenditure. This makes amylin receptors (AMYRs) important targets for potential obesity drugs. Even though drugs that mimic amylin and calcitonin are being produced to treat obesity, it is unknown how their different effects are generated at a molecular level, meaning that drug developers are effectively ‘working in the dark’.
Amylin receptors are made up of two components: the calcitonin receptor, and one of three possible receptor activity-modifying proteins (RAMPs). Each of these RAMPs changes how amylin and calcitonin bind to the complete AMYR.
In an international collaboration, led by by Profs Patrick Sexton and Denise Wootten as part of the ARC Industrial Transformation Training Centre for Cryo-electron Microscopy of Membrane Proteins, cryo-electron microscopy at Microscopy Australia’s Monash facility, the Ramaciotti Centre for Cryo-Electron Microscopy, has been used to understand the structural basis for the binding and the selective action of calcitonin and amylin with the various AMYRs.
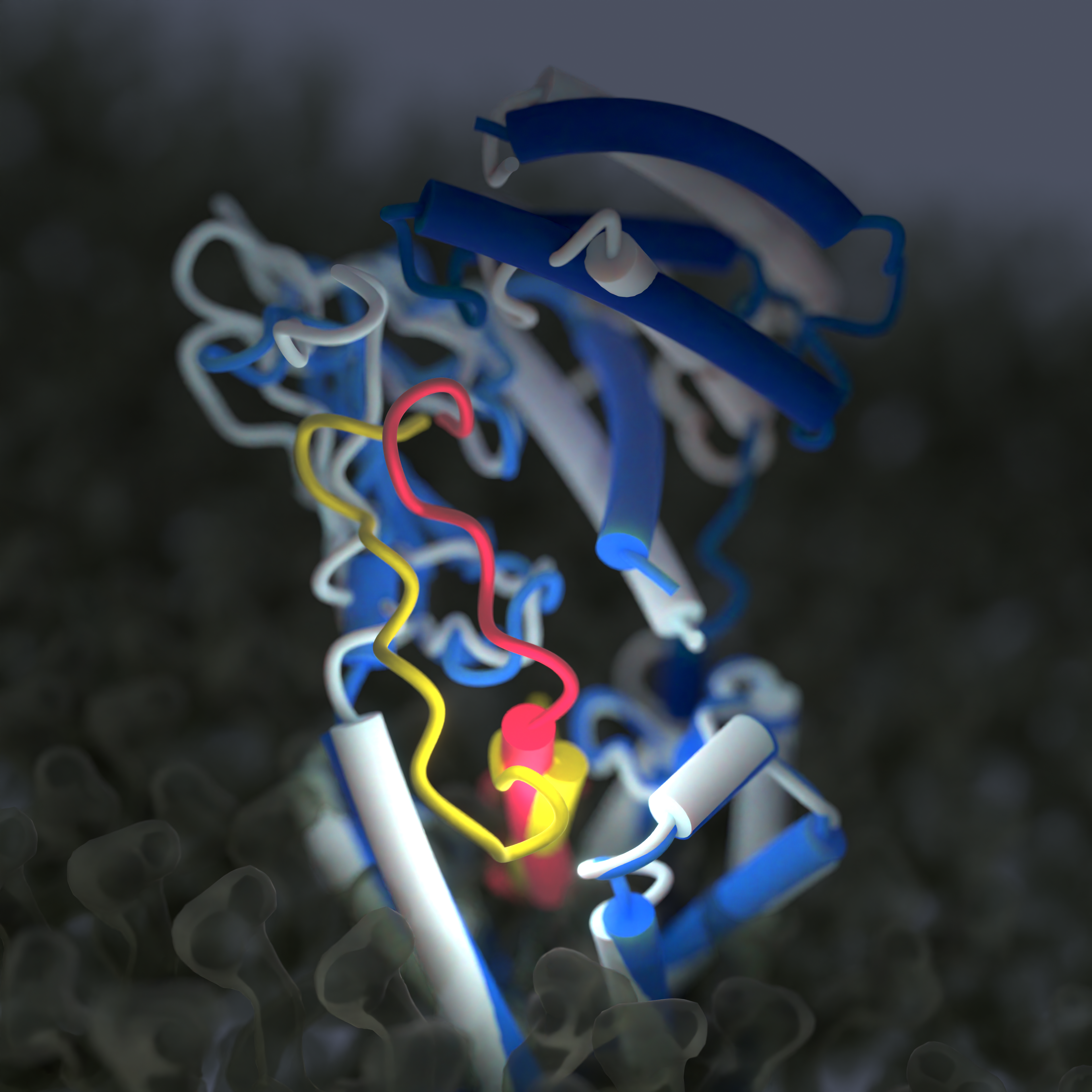
Two overlaid cryo-EM structures showing the differences when amylin-like peptides (yellow) are bound to the receptor (blue) and when calcitonin-like peptides (red) bind to the receptor (light grey). Each receptor is approximately 25nm long. Image created by Dr Sarah Piper @PiperProteins.
Their detailed study, published in Science, has shown how molecules with an amylin structure activate the various receptors differently from molecules with a calcitonin structure. These findings explain some of the unexpected effects seen when newly developed obesity drugs are translated from animal studies to clinical trials. The structural details of hormone binding will enable a much more informed approach to the design of new obesity drugs.
J. Cao et al., Science 2022
DOI: 10.1126/science.abm9609
Each square shows an assembly of cryo-EM images of the AMYR grouped together by orientation, part of the structure reconstruction process. The reconstruction is shown in the right image.
April 5, 2023