Nanosensors can be used as catalysts, to control chemical reactions, and as sensor systems, to detect small molecules in environmental applications. The manufacture of these nanoparticles focuses on maximising their active surface area, and controlling their size and morphology.
Manufacturing noble metal nanoparticles often involves the presence of stabilisers. Stabilisers control the nanoparticle’s size and morphology, but they can cover the surface, blocking the binding molecule required for sensing. This is overcome by dispersing noble metal nanoparticles that are ‘naked’ (devoid of surfactants) on the surface of inorganic 2D materials.
Prof. Colin Raston and his team covered graphene and hexagonal boron nitride (h-BN) nanosheets with ‘naked’ ultrafine nanoparticles of two noble metals – palladium and platinum. Graphene and h-BN offer a high specific surface area, flexible structure, excellent mechanical strength and overall chemical stability, which suit nanosensor production.
Prof. Raston used transmission electron microscopy (TEM) in the AMMRF (now Microscopy Australia) at the University of Western Australia to characterise graphene/h-BN–noble metal hybrids which were around 5 nanometres in diameter – ideal for catalytic reactions. High-resolution TEM and fast Fourier transform identified the characteristic lattice fingerprint for palladium and platinum nanoparticles, confirming the metals’ presence.
These materials can now be used to fabricate devices using a drop-casting technique, also developed by Prof. Raston’s research team. They may also have a role in organic transformations for the pharmaceutical industry.
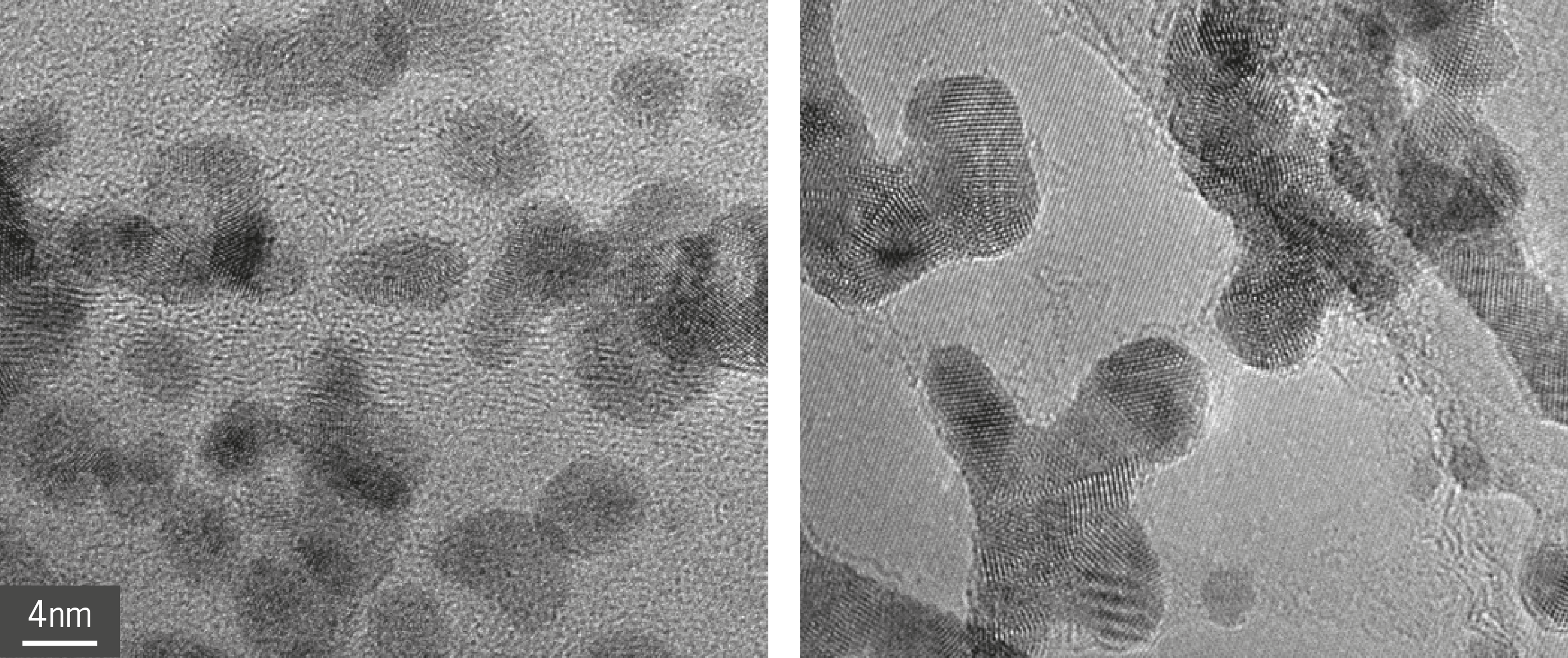
High-resolution TEM image of palladium nanoparticles anchored to graphene (left) and h-boron nitride (right).
Chen et al., Chemical Communications 49(1160), 2013,
High-resolution TEM image of palladium nanoparticles anchored to graphene (left) and h-boron nitride (right).
October 24, 2014