The protein, called LRRC15, sticks to the viruses and creates a barrier, preventing them from entering and infecting cells. It forms part of the body’s innate defense system and could be a target for future anti-COVID treatments.
When SARS-CoV-2, the virus that causes COVID-19, enters the body, the first step in the infection process is for the virus to attach to a protein called ACE2 on the outside of a host cell using it’s spike protein. However, the virus can also bind to LRRC15. When it does this, the virus can no longer bind to ACE2, taking it out of circulation. LCCR15 can also stick to other viruses and this process is likely to be a general mechanism that helps protect the body against viral infection. The researchers, led by Prof. Greg Neely, used confocal microscopy at Microscopy Australia’s University of Sydney facility to help demonstrate this binding.
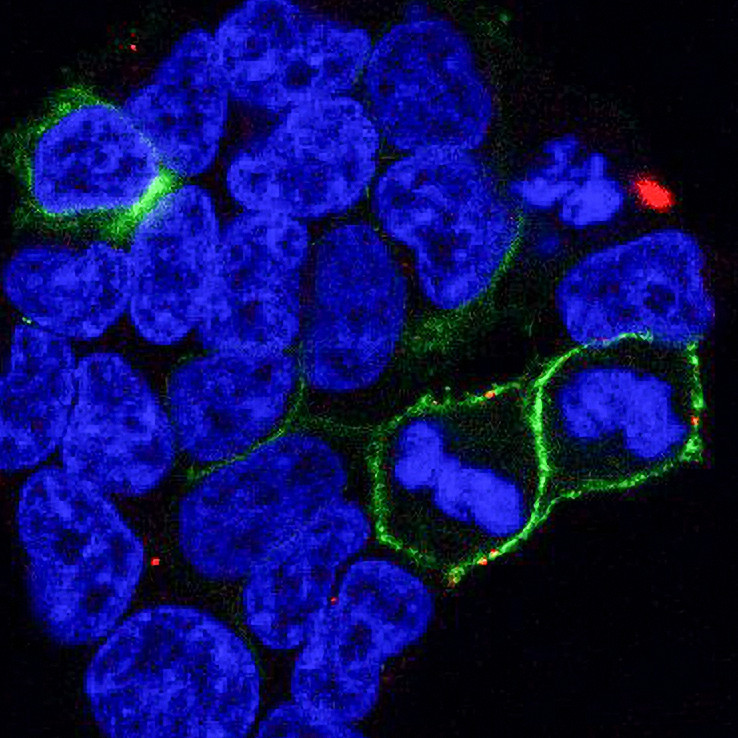
Confocal image of cells from the project showing LCCR15 in green and the SARS-CoV-2 spike protein in red. Taken by Dr Cesar Moreno at the University of Sydney facility.
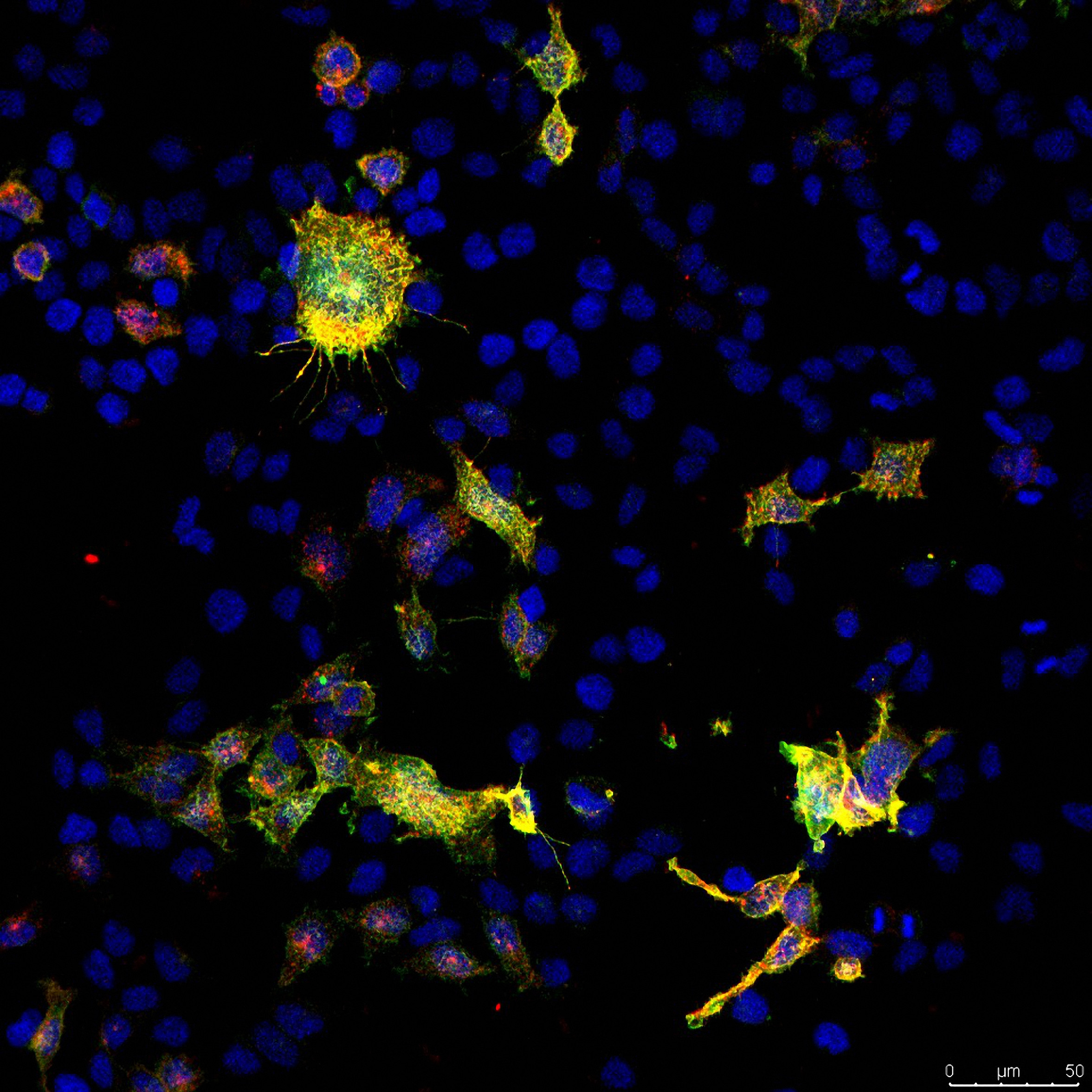
Confocal image from the project showing cultured cells with LCCR15 in yellow and green and the SARS-Cov-2 spike protein in red. Taken by Dr Cesar Moreno at the University of Sydney facility.
LRRC15 is found at places in our body that form protective immune barriers including placenta, skin, mouth, throat and lymph nodes, but it isn’t present in high levels in healthy lungs. However, during COVID-19, the amount of LRRC15 in the lungs increases, where it lines the airways.
Data from these researchers and others found a striking relationship between LRRC15 levels and disease outcome, and out of all the proteins in the blood, LRRC15 levels were the most important for predicting patient outcomes. High levels of blood LRRC15 predict less severe disease, whereas decreasing LRRC15 levels in the blood predict more severe COVID-19.
When cells turn on the LRRC15 gene to start making the protein, this also turns up the production of other antiviral molecules while, at the same time, switching off the production of collagen. Collagen would otherwise lead to scarring (fibrosis) of infected lungs, adding to the debilitating effects of the disease.
One of the lead authors, Matthew Waller said “When we studied how this new receptor works, we found that this receptor also controls antiviral responses, as well as fibrosis, and could link COVID-19 infection with lung fibrosis that occurs during long COVID.”
“The fact that there’s this natural immune receptor that we didn’t know about, that’s lining our lungs and blocks and controls virus – that’s crazy interesting.” Said Prof. Neely. “We can now use this new receptor to design broad-acting drugs that can block viral infection or even suppress lung fibrosis,” Neely said. There are currently no good treatments for lung fibrosis, he said.
Lipin Loo et at., Plos Biology 2023 DOI: 10.1371/journal.pbio.3001967
Confocal microscopy image showing cultured cells with LCCR15 in yellow and green, and the SARS-Cov-2 spike protein in red. Taken by Dr Cesar Moreno at Sydney Microscopy and Microanalysis.
March 2, 2023