From its early research through to clinical trials, Microscopy Australia facilities have been fundamental to the technology’s development. What follows is a journey through the translation process and its multiple strands that have led Ferronova to where it is now.
In 1999, Dr Brian Hawkett and the polymers team at the University of Sydney (USyd) adapted CSIRO’s RAFT polymerisation so it could be applied to emulsions. This was the beginning of a long-term and ongoing collaboration with Dulux for applications in paint technology. Microscopy at the University of Sydney was used to support this work.
“There is no better example of industrial collaboration than this. Dulux has drawn on the important polymer research discovery of CSIRO in RAFT mediated polymerisation and its application to emulsion polymerisation with the assistance of the Hawkett group at The University Sydney. This research collaboration was established in 1999 and has gone on continuously, generating six new patent families.” – Tim Davey, Dulux Australia research manager, 2016 in response to their successful Linkage project.
In addition to ongoing work with Dulux, the Hawkett research team started working with Sirtex on cancer applications, bringing their expertise in polymer stabilisation to the realm of medical micro- and nanoparticles. In 2011 they were granted a key patent for administrable compositions using block copolymers.
In parallel with Dr Hawkett’s work in Sydney on polymer stabilisation, Prof. Benjamin Thierry at the University of South Australia was collaborating with surgeons in gastric, oesophageal, colorectal, and head and neck cancer, and, as part of this program, developed and patented a sensor for detection of magnetic nanoparticles. Meanwhile, in New Zealand, Prof. Richard Tilley had developed magnetic nanoparticles and commercialised them through a previous spin-off company. Through a working collaboration on their complementary technologies, Profs Tilley and Thierry set up Ferronova in 2016 bringing together intellectual property from the University of South Australia and Victoria University in New Zealand, with seed investment provided by Powerhouse Ventures. Their aim was, and remains, to use magnetic nanoparticles as tracers to track the spread of cancer to the lymph nodes, something that would be of great benefit to surgeons particularly those operating in complex areas of the body such as the stomach, oesophagus, head and neck.
In 2017, Ferronova started collaborating with Prof. Hawkett and in 2019 licensed his above-mentioned polymer stabilisation technology to enhance the biocompatibility and stability of their magnetic nanoparticles. This combination of technologies enabled Ferronova to move forward so that over the 2016–2020 period they successfully completed a series of preclinical validation studies of their first product, FerroTrace®, including large animal feasibility and optimisation studies enabled by the National Imaging Facility.
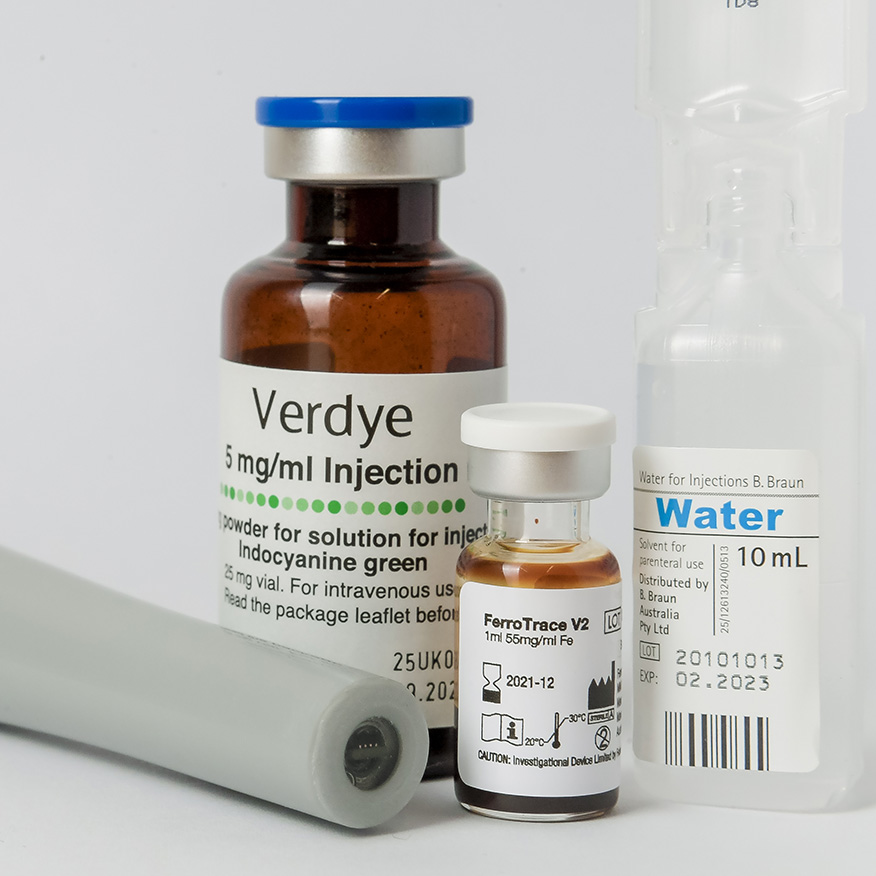
Through successful funding rounds and grants, Ferronova has got to the position where it completed its first clinical trial of FerroTrace® on 15 patients with oral cancer at the Royal Adelaide Hospital in 2022 and has now progressed into other cancers with a total of 40 patients having been administered their nanoparticles. Microscopy Australia is still involved, testing every batch of nanoparticles Ferronova produce.
Ferronova continues to collaborate with Profs Hawkett, Tilley and Thierry, and has established collaborative programs for new medical applications of the technology involving Siemens and other research partners in cancers where there are significant unmet medical needs including glioblastoma and pancreatic cancer.
Profs Hawkett, Tilley and Thierry are now all Scientific Advisors of Ferronova and continue to be heavily involved in the technology’s ongoing development as it moves towards FDA approval and release onto the market.
September 20, 2024