However, these devices often don’t integrate well with host tissue and may need replacing through revision surgery. This causes significant pain and suffering, as well as being an economic burden. Biomaterial coatings can mitigate these problems by masking the implanted devices, mimicking the surrounding tissue, and allowing successful integration with the body.
Ideal surfaces interact seamlessly with host tissue through a surface covered with appropriate proteins. Although whole proteins can improve device biocompability, they don’t always survive the required sterilisation and can bring the risk of infection. Prof. Marcela Bilek, Dr Behnam Akhavan, and Mr Lewis Martin at the University of Sydney are designing synthetic short protein segments, called peptides, to recapitulate the function of specific proteins. Their small size can make them more resilient to sterilisation. The main challenge is ensuring they attach to device surfaces at appropriate densities and orientations. The team have discovered how to control peptide attachment by using applied electric fields. The charged peptides interact with the surface in a preferred orientation and density when an electric field is applied.
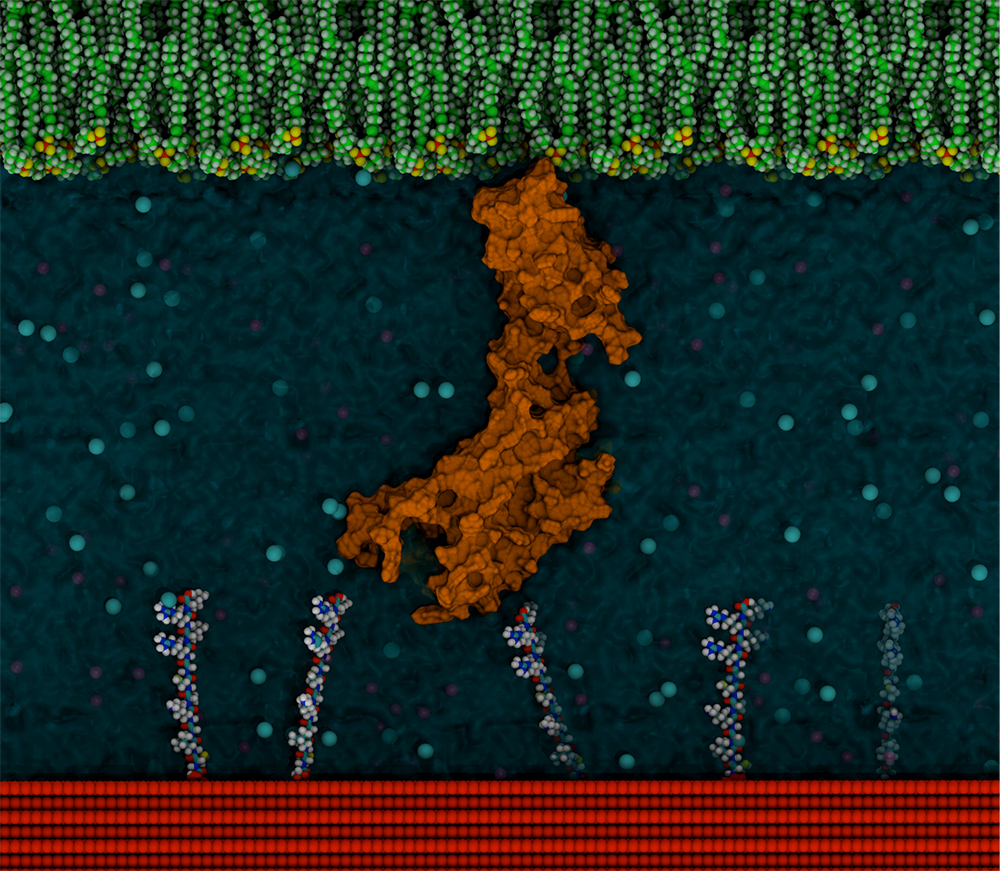
Illustration of a cell surface receptor protein (orange) on the outside of a cell (green) coming into contact with a peptide-functionalised surface (red). The peptides on the surface can bind to the protein and influence the cell’s behaviour.
The team uses the AMMRF (now Microscopy Australia) flagship time-of-flight secondary ion mass spectrometer at the University of South Australia. This can measure the atoms on the top-most surface of the device, enabling a detailed understanding of the distribution and orientation of the attached peptides. This new knowledge is being used to develop strategies to create a new generation of biologically functional surfaces for implantable devices.
November 27, 2016