By taking advantage of the Microscopy Australia Technical Voucher Fund, established with the support of MTPConnect, they were given easy, discounted access to our sophisticated microscopes and expert technical help. This enabled important R&D work for the company.
“Engaging with Microscopy Australia, through their facility at UNSW, we have been able to validate the physical makeup of some of our novel investigational formulas. This work, supported by a Microscopy Australia Technical Voucher, was very timely, as we were able to use world-class resources, including the new Talos Arctica cryo-EM instrument to image our samples.
“Little Green Pharma is continuing to engage with academic research partners to develop novel approaches to delivering cannabinoid-containing formulas as efficiently as possible for maximum clinical effect. We intend to maintain our association with Microscopy Australia as we are committed to continuing R&D in the medicinal cannabis space,” said Lilly Bojarski, Medical Science Liaison (NSW) for Little Green Pharma.
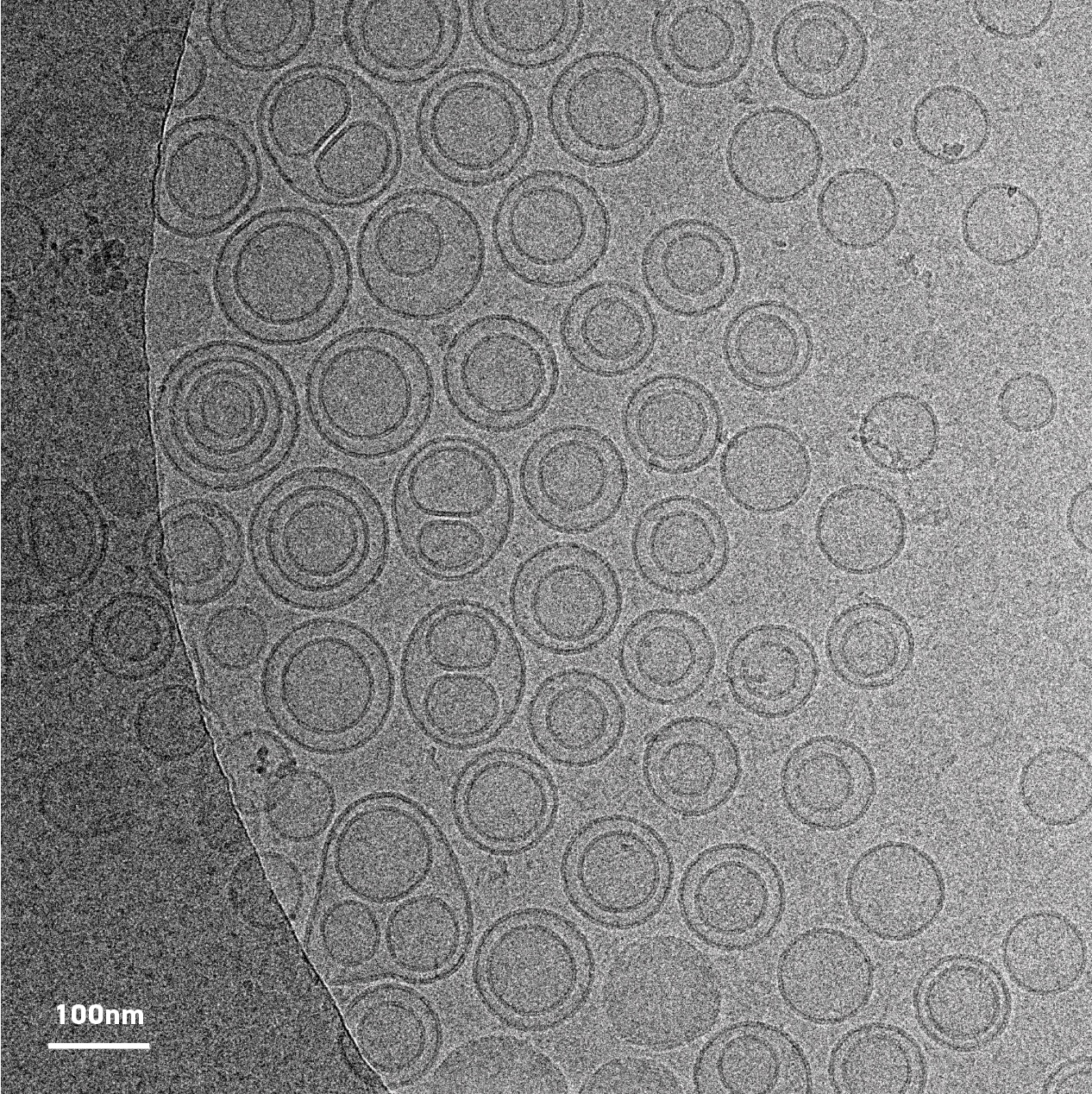
Cryo EM image of cannabinoid-containing liposomes taken at Microscopy Australia’s UNSW facility
“We have subsequently been granted another voucher to continue our research. This will enable LGP to monitor our formulas for any degradation during various storage conditions,” said Damian Wood, Head of Pharmaceuticals.
He continued “We are currently producing our LGP Classic 10:10 oil oral liquid formulation, where each mL contains 10mg THC and 10mg CBD derived from medicinal cannabis whole plant extract. THC and CBD in a 1:1 ratio is a common starting point for practitioners. We are also continuing to expand and improve our range of available formulas by engaging with academic and other partners to conduct pre-clinical and clinical development R&D work with new medicinal cannabis formulations, including many for which we have secured IP protection.”
“While ensuring supply of our currently available formula (to prescribing physicians for patients who meet the criteria set out by their state health authority regulations and via the TGA’s Special Access Scheme B) we are also committed to developing new pharmaceutical-grade medicinal cannabis formulations of quality and efficacy for Australians with unmet clinical needs.” said Fleta Solomon, CEO.
July 19, 2021