It also saw the renewal of Microscopy Australia’s ACMM micrograph competition. With 38 entries the competition was steep, making the job of our judges very difficult!
After much deliberation, we had the pleasure to announce three prizes, first place went to Dr Matthew Field, and the runner up awards went to Angus Rae and Lisa Griffiths. In agreement with the judges, the people’s choice award also went to Dr Matthew Field, whose image was featured on the cover of the most recent edition of the Australian Microscopy and Microanalysis Society newsletter. Each of the microscopists have supplied some information on their winning micrograph below.

This image of a vertically oriented carbon nanotube array was taken using an FEI Scios Dualbeam SEM. By assigning each of the secondary electron detectors (T3, T2 & ETD) a different colour (red, green and blue) and combining them, this composite image was formed.
Back in the good(?) old days, it was considered to be fairly challenging to collect ‘colour SEM’ images with multiple detectors. Not only would you have to play nice with a grumpy microscopist and convince them that it was actually a good idea to attach 3 secondary electron detectors (typically ETDs) to the microscope, but then there was also the challenge of extracting the signal from them all simultaneously.
For those artistically inclined, and those who lack the time for a large scale colouring-in project, we can be thankful that nowadays most SEMs have many in-chamber detectors, in-column detectors and segmented detectors. Simultaneous acquisition from multiple detectors/segments is a breeze and these images can easily be combined to form works of artistic beauty to rival that of Monet, Picasso (or Banksy, if you prefer).
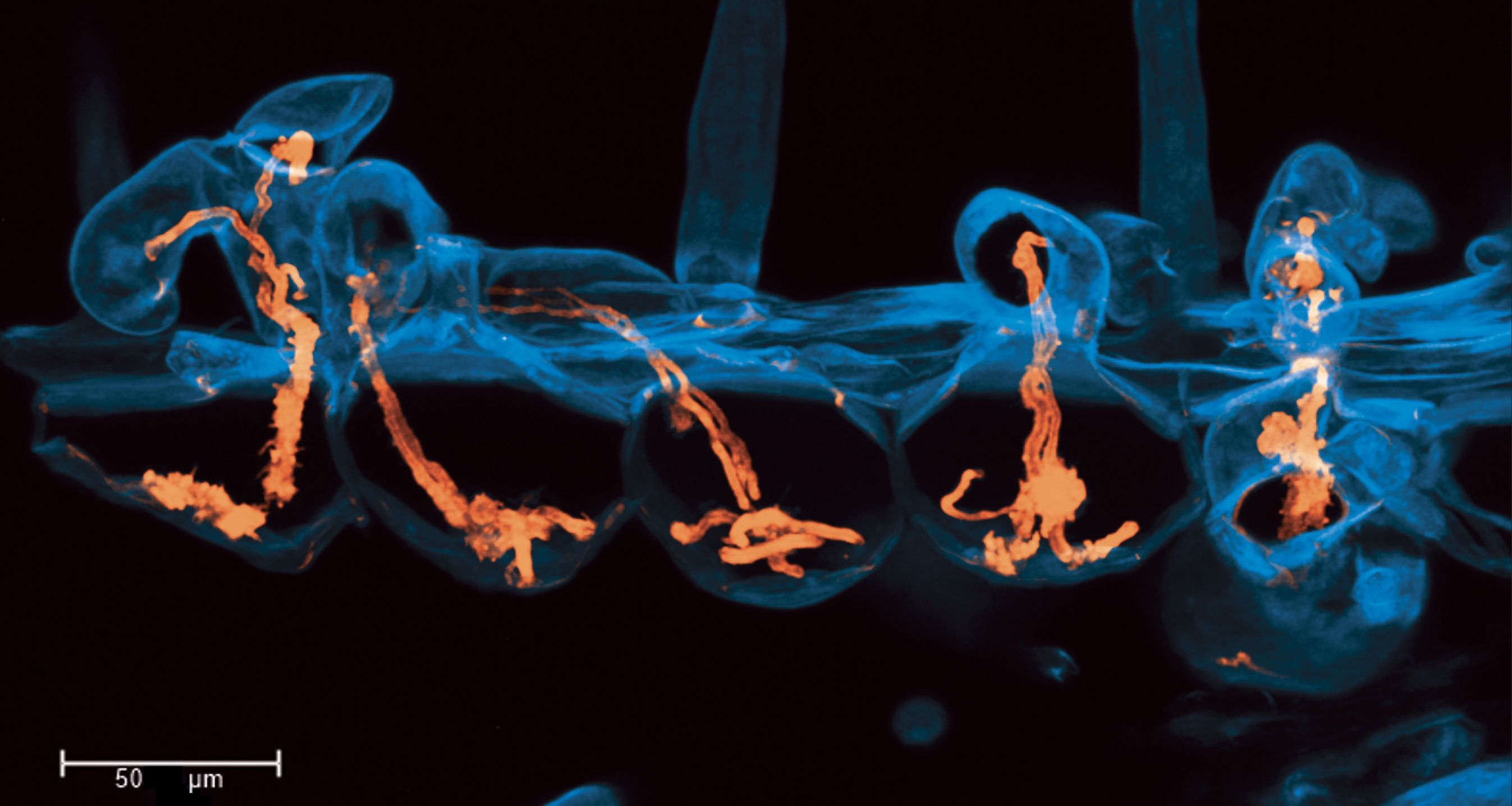
The image shows hyper-infection of symbiotic infection threads (orange) in root hair cells (cyan) of the model legume Medicago truncatula. Infection threads are tubes of plant cell wall that transport symbiotic nitrogen fixing rhizobacteria into root nodules on legume roots. While rarely occurring in adjacent cells of the wildtype, this mutant line is hyperinfected, with too many infection threads.
These five infected cells were imaged in a whole cleared root by staining with calcofluor white and rhodamine-123 PAS, shown in cyan and orange respectively. Image is a max projection of a confocal z-stack captured on a Leica SP8, HC PL APO CS2 40x/1.10 WATER objective.
We have published the method I used in A.E. Rae et al. New methods for confocal imaging of infection threads in crop and model legumes. Plant Methods 17, 24 (2021) DOI: 10.1186/s13007-021-00725-6
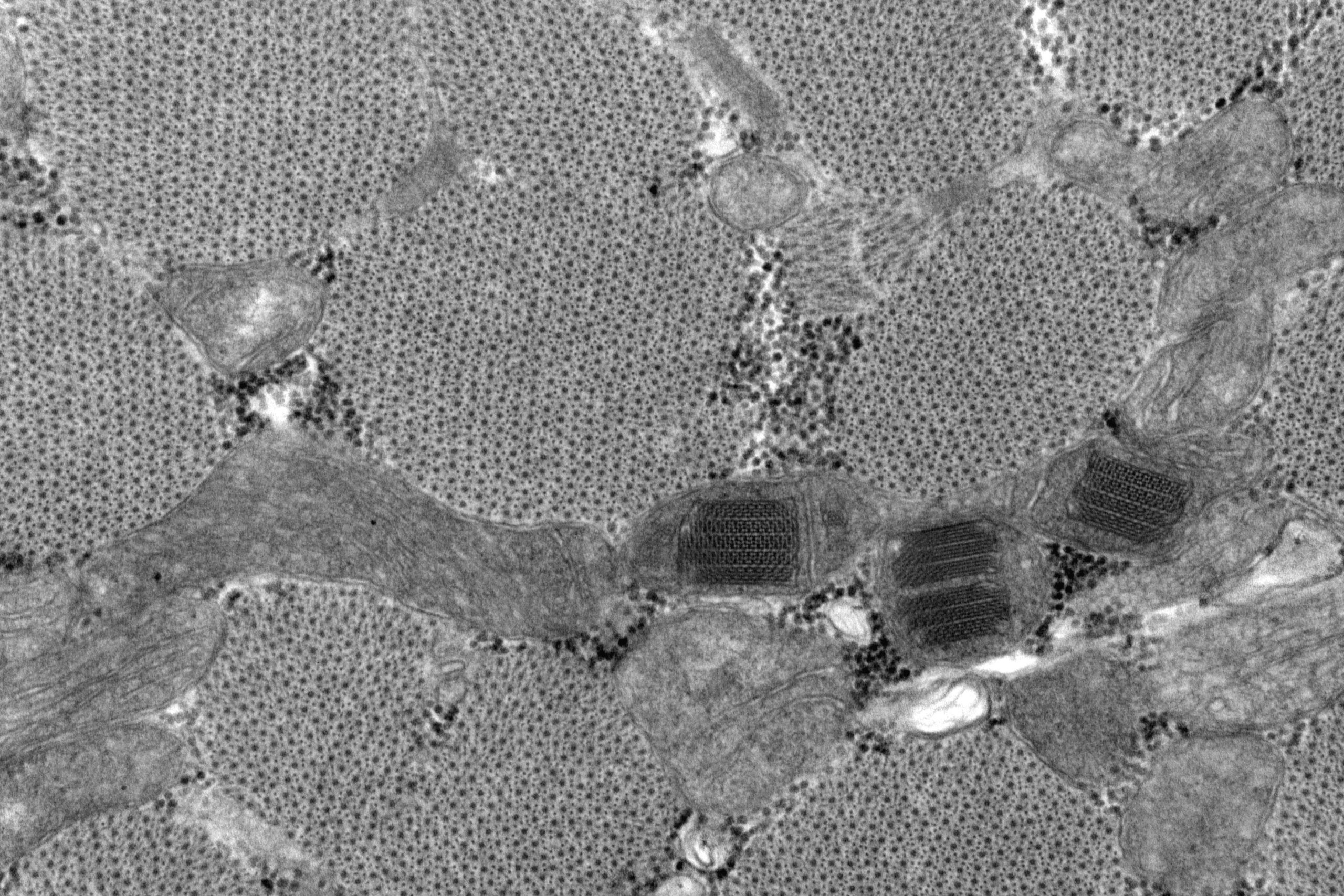
The image shows mitochondrial paracrystalline inclusions in skeletal muscle (transverse). This is from a Vastus Lateralis biopsy of an 81 year old male who had been on statins in the past, although not at the time of biopsy. He presented with progressive myopathy. The crystalline inclusions were noted but formed part of the overall non-specific findings.
It was fixed in 2.5% buffered Glutaraldehyde, prior to being cut up for processing and resin embedding in EM. Cutting and embedding are done with orientation in mind, producing longitudinal and transversely oriented blocks for screening. The processing involves an Osmication step, dehydration in graded alcohols, transition through Propylene Oxide and Prop Ox/resin gradients until impregnation in pure resin. When blocks are polymerised, thick (0.5-1) micron sections are cut using a glass knife for glass slides and stained with methylene blue. After selection of most appropriate block/s, thin sections are cut on a diamond knife and further stained with Uranyl Acetate and Lead citrate before viewing on a JEOL 1400 TEM, and images captured with a GATAN Orius 11Mp digital camera. Some post capture editing took place to improve contrast where needed.
March 20, 2023