We’re partnering with our NCRIS colleagues at the National Imaging Facility to bring you stories illustrating the world-leading impact of Australia’s imaging community, supported by our national research infrastructure.
Through open access to state-of-the-art expertise, equipment, tools, data and analysis, we’re proud to empower Australian researchers from a broad range of disciplines, from engineering to medical science, to address global challenges. The power of advanced imaging technology is driving solutions for Australia’s strategic science and research priorities, fostering innovation, supporting industry and contributing to our health and wellbeing.
Over this week we will be featuring new bioimaging techniques being developed by our experts, and the real world impact these techniques are having. We will also feature new and exciting biomedical research enabled by our facilities. Keep an eye out on our socials, or head over to the National Imaging Facility website for more Australian imaging.

Photo from the 2022 Cryo-EM masterclass hosted by A/Prof. Ramm
The imaging of molecules, cells, and tissues are central to biomedical research and clinical practice. Advancing imaging tools and accelerating their use will help drive breakthroughs in curing, preventing, or managing disease. To this end, the Chan Zuckerberg Initiative have funded A/Prof. Georg Ramm, director of our Monash facility, the Ramaciotti Centre for Cryo Electron Microscopy, to develop a medical imaging technique for tissues: correlative cryo-fluorescence microscopy and electron tomography.
This project aims to develop workflows for cryo-tomography in tissues so that researchers can study tissues’ molecular architecture in health and disease, and to then teach these workflows to fellow researchers. As part of this, A/Prof. Ramm has delivered two Microscopy Australia masterclasses, one on cryogenic electron microscopy and one on cryogenic correlative light and electron microscopy.
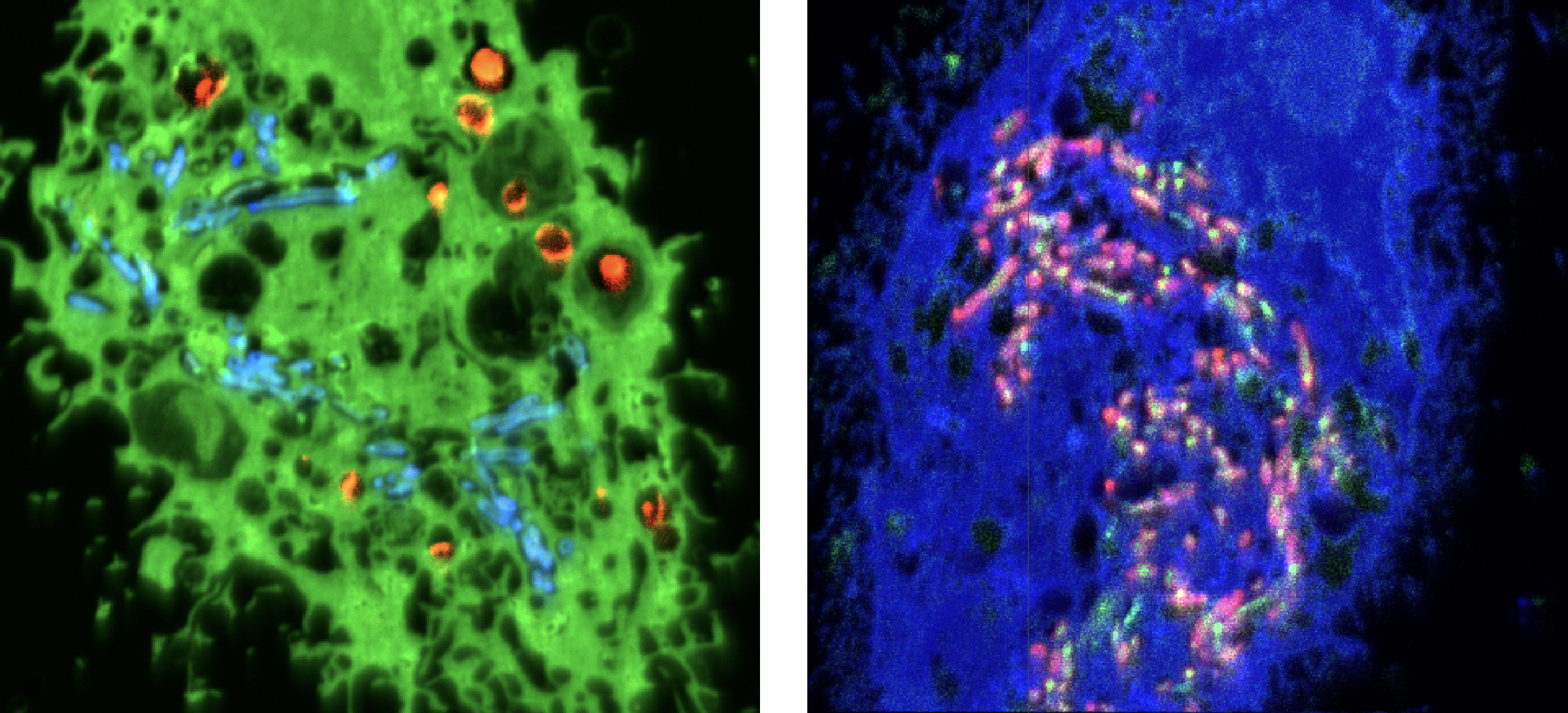
NanoSIMS images of two tuberculosis-infected cells from the study.
Tuberculosis may not be the curse it was before the era of antibiotics, but treatment is still long and tedious and, with the emergence of antibiotic-resistant strains, the tuberculosis (TB) threat is rising again, killing 1.6 million people worldwide in 2021. In order to develop better treatments, researchers need to understand how current antibiotics work to kill the TB bacteria, Mycobacterium tuberculosis, within our cells.
Our expert staff at the Centre for Microscopy, Characterisation and Analysis, working with researchers from the University of Western Australia, Hong Kong, the UK and Switzerland, have developed a new imaging method that combines correlative light, electron and ion (nanoSIMS) imaging that allows researchers, for the first time, to see where the bacteria and antibiotics are located in the cells. This has allowed them to decipher how different intracellular micro-environments affect the action of the TB drugs.
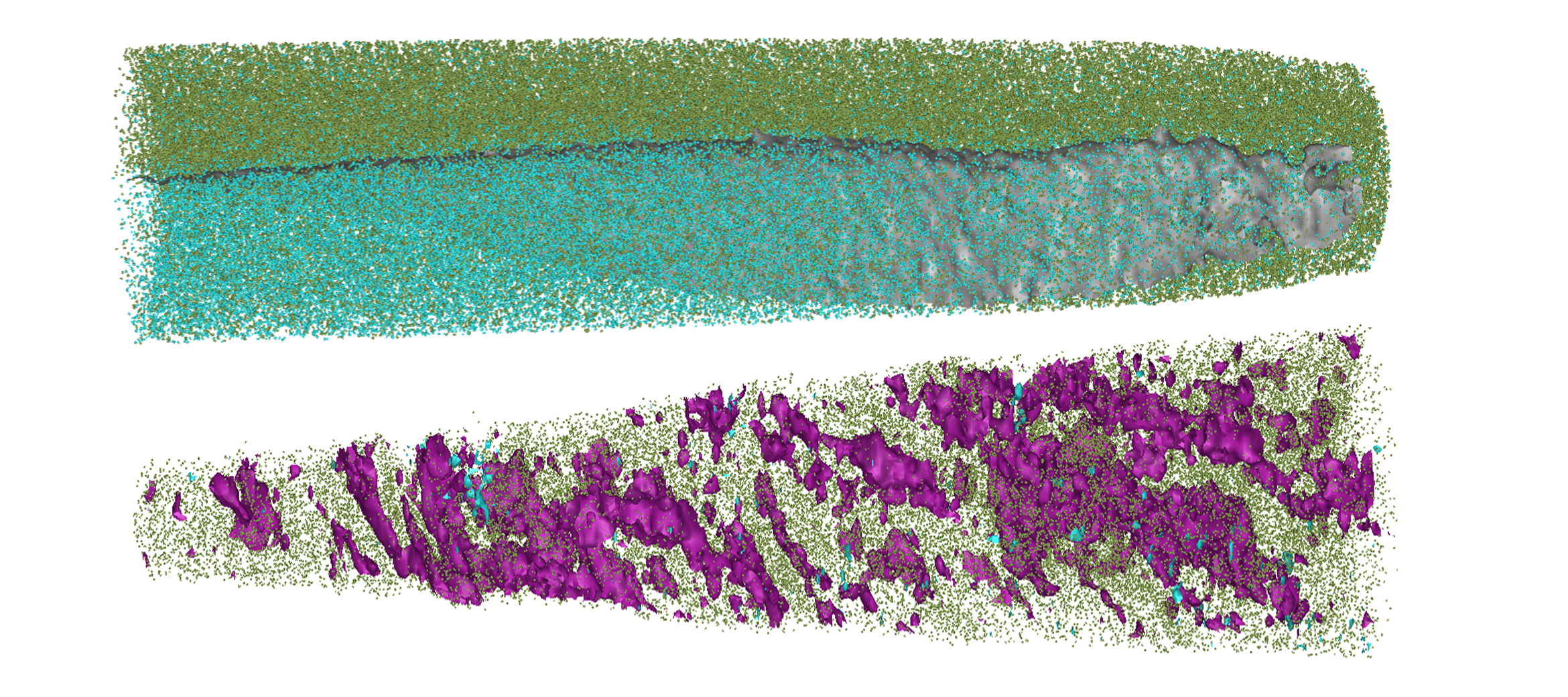
APT reconstructions showing the placement of atoms within normal bone (bottom) and the implant (top) after being implanted in a sheep for 12 months. Purple is carbon, green is calcium and turquoise is aluminum. At it’s thinnest point, left is~20 nm across, while right is ~30 nm across
Atom probe tomography is a high-end imaging technique that provides 3D data on the locations of atoms within a solid sample. Traditionally this has primarily been used by engineers and materials scientists to better understand the atomic structure of metals, ceramics and electronic components.
Recently, in collaboration with CAMECA, who produce atom probes, our expert Dr Natalie Holmes has been developing techniques that allows the atom probe to be used for biomedical applications. Currently, they are harnessing atom probe tomography to understand the biocompatibility of synthetic bone implants and map how the implant is interacting and exchanging material with new bone tissue at the atomic scale. Our facilities were also used by Prof. Hala Zreiqat to develop the bone scaffold being studied.
Read more about the bone scaffold project
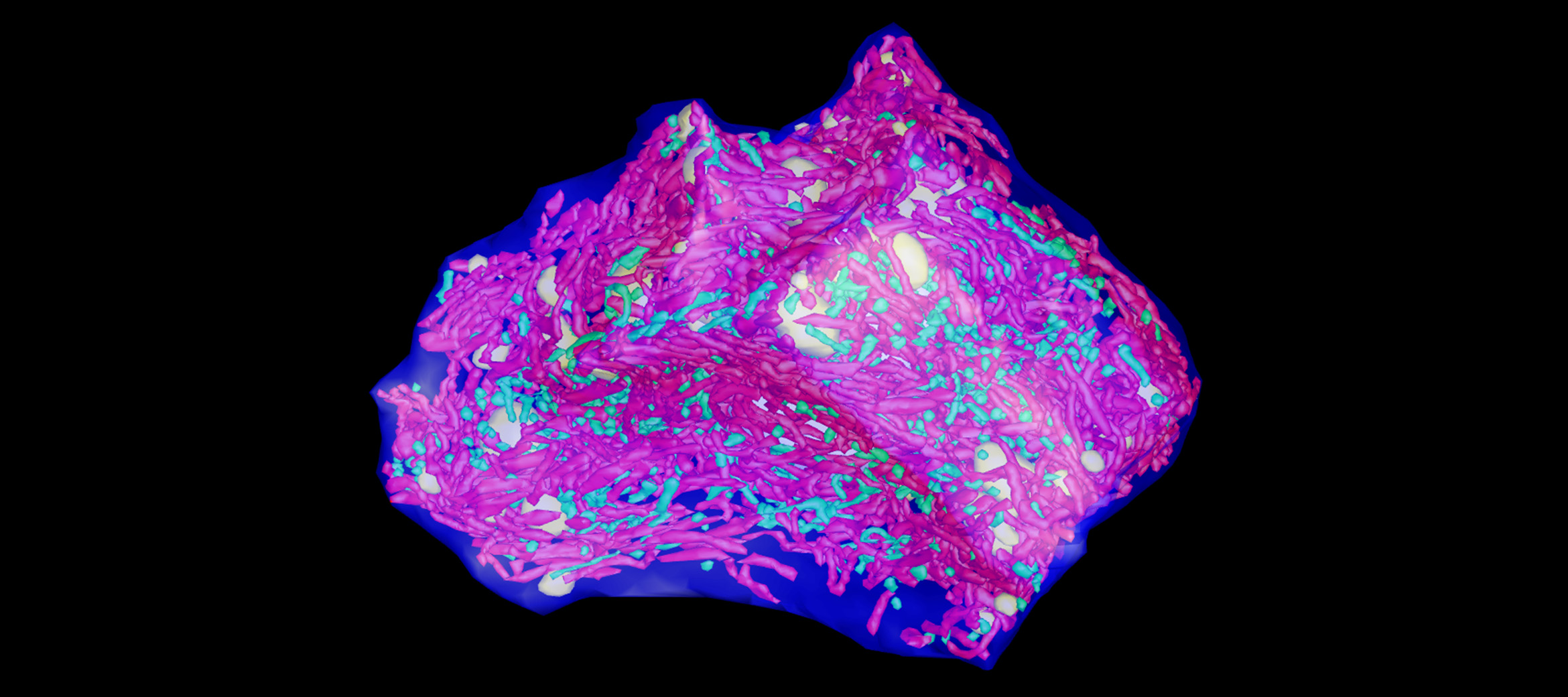
3D image of a liver cell with the normal mitochondria in green and the giant mitochondria in red generated by transmission electron tomography.
Microscopy Australia’s expert scientists, Sydney Microscopy and Microanalysis facility director Prof. Filip Braet and Dr Gerry Shami, used advanced 3D multimodal microscopy to reveal the structure of the giant mitochondria found in cells from patients with non-alcoholic fatty liver disease (NAFLD). The 3D nature of the techniques used, made it possible to measure mitochondrial surface area and volumes as well as understanding the structures and arrangements of the inclusions. This led the researchers to suggest a model where mitochondria fuse to form the giant mitochondria seen in the disease state.
The impact of the paper is clear: out of around 2,000 cell and molecular biology papers in Scientific Reports in 2021, this paper was the 30th most downloaded.
January 23, 2024