Despite increasing vaccination coverage, the WHO estimates there are still 1.5 million deaths each year from vaccine-preventable diseases (VPD). This is due to a series of issues, including:
Prof. Mark Kendall, who started this work at the University of Queensland (UQ), has developed a device, dubbed the HD-MAP, that could revolutionise vaccine delivery. The HD-MAP, previously known as the Nanopatch™, is a one square-centimetre silicon patch covered with thousands of vaccine-coated micro-projections. These pass through the skin’s outer layer to deliver the vaccine directly to the immune cells in the skin. These cells mount a more efficient immune response than muscle, which vaccines are traditionally delivered into. Tests showed that only 1-2.5% of the dose currently used for influenza and polio vaccines was needed to elicit the same immune response. The HD-MAP now being commericalised by spin-out Vaxxas.
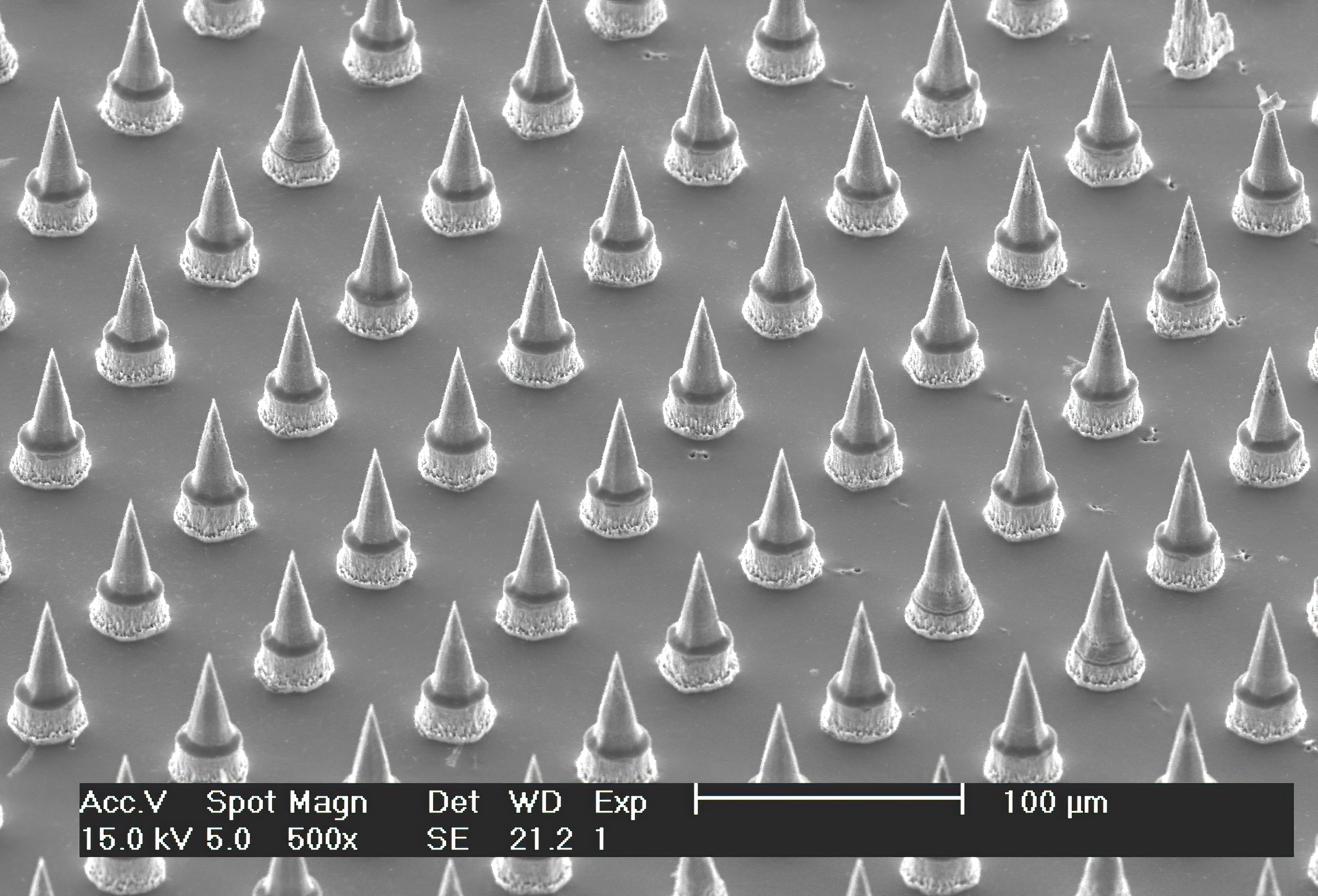
Scanning electron micrograph of the Nanopatch™
Throughout its development, pre-clinical and current clinical trial phases, Prof. Kendal and Vaxxas have used microscopes at Microscopy Australia’s UQ facility to monitor the design, production and functionality of the HD-MAP. Our microscopes feature in over 20 HD-MAP publications and have been used in its development every year since 2009.
Our UQ facility was also used in the development of several vaccines by UQ researchers that are being trailed with the HD-MAP. Read more about how our facilities were used in the development and trials of a HD-MAP-based dengue fever vaccine, and ongoing trials of a needle-free COVID-19 vaccine. With success in several clinical trials including polio, Vaxxas is now working on trials for seasonal influenza, pandemic influenza and measles-rubella.
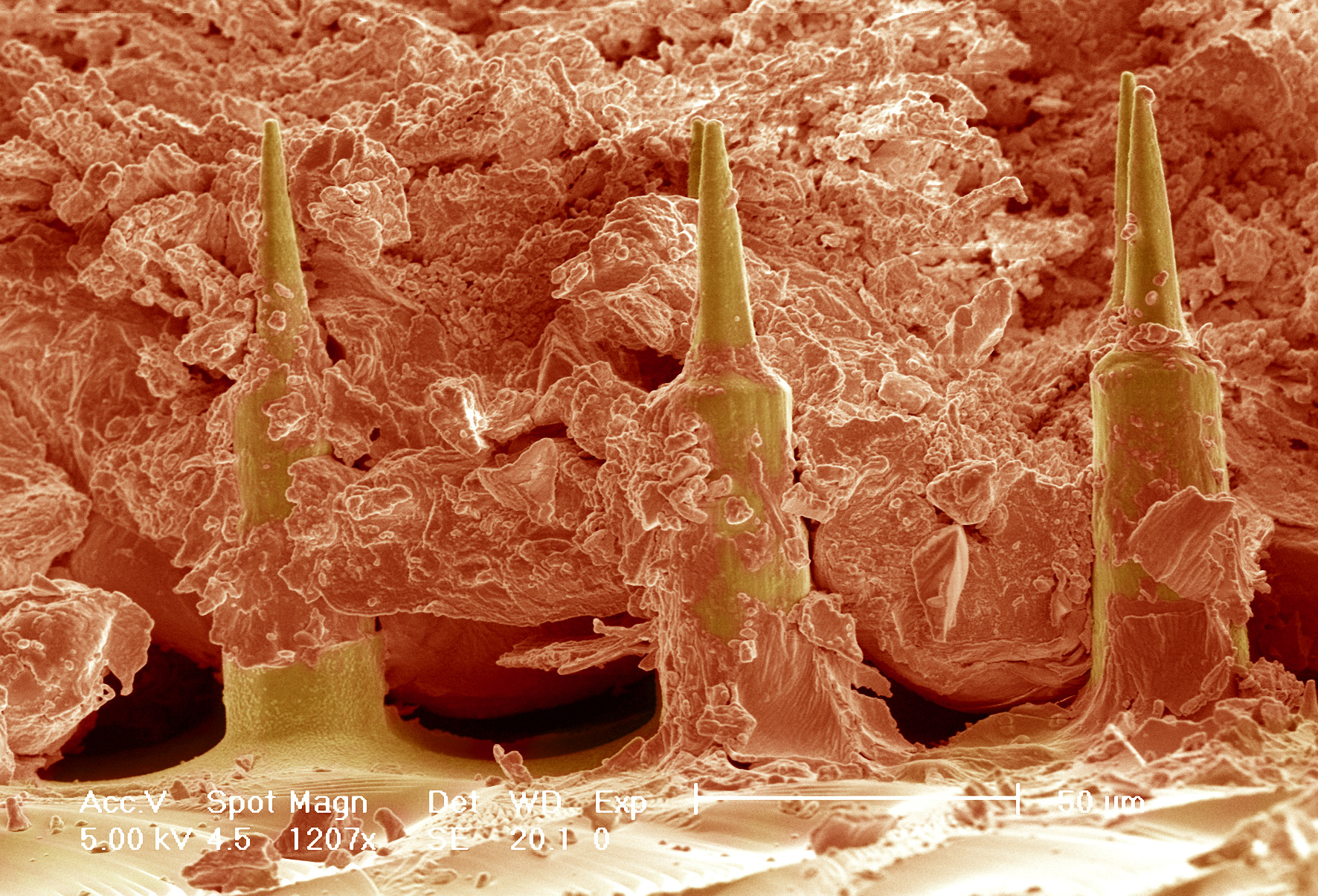
False coloured Scanning electron micrograph of the Nanopatch™ (yellow) in skin (orange)
The HD-MAP is a game changer for global vaccination programs for several reasons:
Vaxxas has secured both global partnerships and investment, attracting over $35 million in initial funding along with a deal with world-leading vaccine producer, Merck, for further R&D and trials. Bill & Melinda Gates Foundation have provided two grants totalling $14.25 million for preclinical development and trials for measles and rubella vaccinations. In 2024 Vaxxas received a global licence for a next-generation vaccine antigen for Respiratory Syncytial Virus (RSV) from the United States National Institutes of Health, opening the door for clinical trials.
Recently, Vaxxas opened a the 5,500m² Vaxxas Biomedical Facility, a first-of-its-kind manufacturing site designed to support the scale-up of the HD-MAP for late-stage clinical trials and first commercial products. Vaxxas currently employs 130 people in its Queensland operations, and is planning for an increase to 200 employees over the next three to five years.
The Australian National Fabrication Facility has also supported this work.

A vaccine being delivered using the Nanopatch™
Colour-enhanced scanning electron micrograph of Vaxxas' High-Density Microarray Patch (HD-MAP, previously Nanopatch™) (green) coated in vaccine (yellow).
September 22, 2020